Overview
RinuaGene Biotechnology Co., Ltd. has advanced its self-developed mRNA therapeutic vaccine RG002 for HPV-related tumors and precancerous lesions into clinical development.
A research paper titled "Development of an mRNA-based therapeutic vaccine mHTV-03E2 for high-risk HPV-related malignancies" has been published on Molecular Therapy. RinuaGene and the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College, are the joint corresponding institutions for this article. https://doi.org/10.1016/j.ymthe.2024.04.036.

mHTV-03E2 encompasses six antigens from HPV types 16 and 18. This antigen design not only targets the two most clinically significant high-risk HPV types but also moves beyond the common approach of solely using E6/E7 as antigens. By addressing both E6/E7-driven and non-E6/E7-driven tumors, this design offers a potentially optimal solution for treating HPV16/18-related precancerous lesions and tumors. Additionally, the incorporation of the E2 protein, which plays a crucial role in viral replication and early lesions, significantly expands the clinical applicability of this antigen design.
The early research data underscores RinuaGene's innovative design strategy for the mRNA therapeutic vaccine (RG002) targeting HPV-related diseases. Through a multi-antigen combination and integrated antigen presentation enhancement components, the design can induce robust cellular immunity and antibody-dependent cellular cytotoxicity (ADCC), among other immune responses and their synergistic effects. This approach achieves excellent immunological and anti-tumor outcomes while greatly expanding the potential patient population for indications. Excitingly, experiments have also shown that this candidate therapeutic vaccine can significantly enhance the response of cold tumor models to immune checkpoint inhibitors.
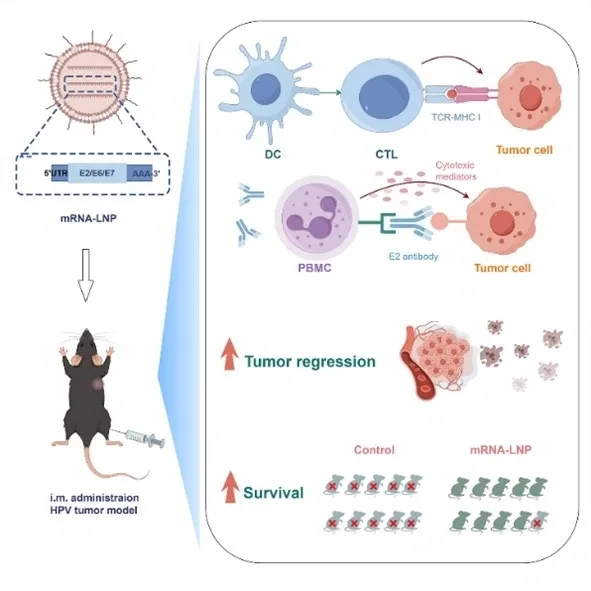
Building on the preliminary research presented in this paper, RinuaGene has fully optimized its proprietary delivery system, sequence design, regulatory elements, and formulation through various patented technologies, leading to the development of the HPV therapeutic mRNA-LNP vaccine RG002. In 2023, RG002 received FDA approval for clinical trials aimed at treating cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3) associated with HPV types 16 and/or 18, making it the first mRNA-LNP therapeutic vaccine globally to gain approval for clinical trials for this indication.
RinuaGene will advance the clinical development of related indications worldwide, actively exploring both monotherapy and combination treatment strategies for HPV infection-induced precancerous lesions and various malignancies, addressing the significant clinical need for HPV-related disease treatments and benefiting patients globally.
About RG002
HPV infection, particularly with HPV types 16 and 18, is the primary cause of precancerous lesions and cervical cancer. The persistent progression of cervical precancerous lesions ultimately leads to the development of cervical cancer, which is the fourth leading cause of malignancy threatening women's health worldwide. Surgical intervention remains the primary treatment for cervical precancerous lesions; however, it carries several risks, including significantly increased rates of preterm birth, miscarriage, and high recurrence rates. Moreover, HPV infection is directly associated with oropharyngeal cancer, anal cancer, as well as cancers of the vagina, penis, and vulva.
Although preventive HPV vaccines are available, the limited vaccination coverage, existing missed vaccination opportunities, and the prevalence of infected populations continue to pose a broad threat from HPV-related tumors (with over 850,000 new diagnoses each year) and high mortality rates (with nearly 450,000 new deaths each year). Given that no therapeutic vaccine targeting HPV-related precancerous lesions and tumors has been approved globally, the successful development of RG002 holds promise for bringing hope to patients suffering from HPV-related diseases in China and worldwide.
About RinuaGene
RinuaGene is dedicated to developing innovative drugs and vaccines based on mRNA technology to meet significant unmet clinical needs globally. The company possesses comprehensive capabilities for the independent research and clinical production of mRNA drugs and vaccines, with four major technology platforms: linear mRNA, circular RNA, LNP targeted delivery, and CMC. RinuaGene will continue to advance the comprehensive accumulation of intellectual property for its pipelines and technology platforms, establishing a solid global patent landscape.
The company remains committed to an innovative, open, collaborative, and mutually beneficial corporate culture and welcomes multifaceted cooperation in product development, combination therapies, technology platforms, and novel delivery systems to jointly promote the development and application of RNA technology across various fields.