December 04, 2024
RinuaGene's HPV Therapeutic mRNA Vaccine Received IND Clearance from China CDE
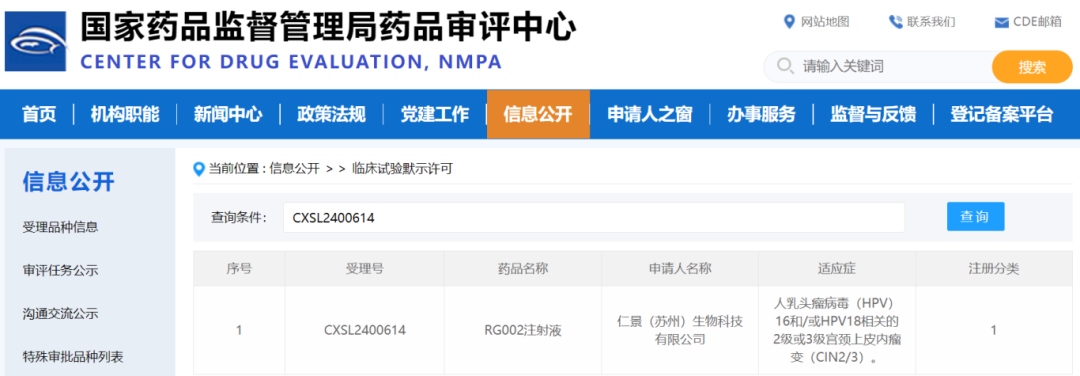
CDE officially announced the clinical trial approval for RG002 Injection.
Read More

October 16, 2024
Advanced Science | RinuaGene Published Data on Novel mRNA LNP Delivery System
RinuaGene publishes novel lipid (4S)-KEL12 paper in Advanced Science.
Read More

May 16, 2024
Molecular Therapy | RinuaGene Published Early Research Data of HPV Therapeutic Vaccine
RinuaGene advances HPV mRNA candidate RG002 into the clinical development.
Read More

November 06, 2023
RinuaGene's HPV Therapeutic mRNA Vaccine Received FDA IND Clearance
RinuaGene has received IND approval from FDA for proprietary HPV-related therapeutic mRNA vaccine.
Read More